Assuming 100 Dissociation Which of the Following Compounds
3 Assuming 100 dissociation which of the following compounds is listed correctly with its vant Hoff factor A CaCIO32 i 2 B NH42SO4 i 3 C NH43PO4 i 5 D Urea i 2 E Sc2SO43 i 6 Answer. Solution for Assuming 100 dissociation calculate the freezing point T and boiling point T of 313 m KPOaq.
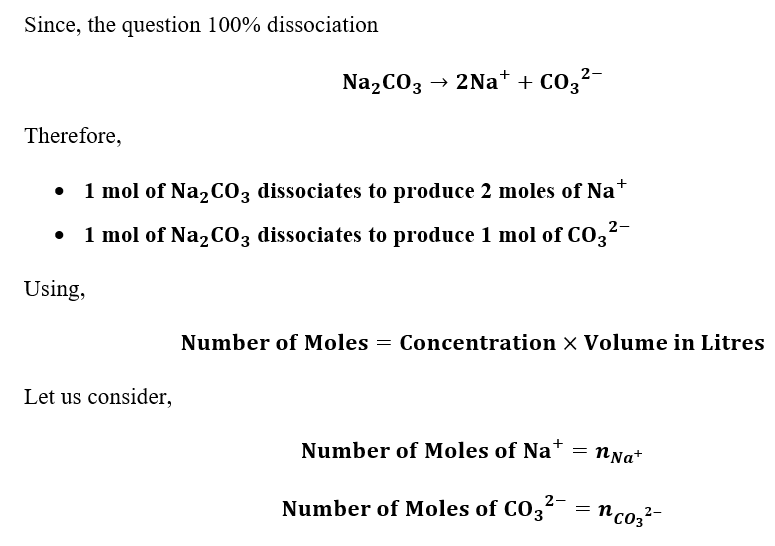
Answered Assuming 100 Dissociation What Is The Bartleby
4 A solution of chloroform CHCl3 and acetone CH32CO exhibits a negative deviation from Raoults law.

. A solution is made by dissolving 00450 mol HF in 100 kg of water. An ionic crystal lattice breaks apart when it is dissolved in water. Think of the number of ions that each compound is made of is it 2 3 4 D Al2SO43 i 4.
Assuming 100 dissociation which of the following compounds is listed incorrectly with its vant Hoff factor i. B NH 4 2SO 4 i 3 C NH 4 3PO 4 i 5 D Urea i 2 E Sc 2SO 4 3 i 6. Dissociation is the separation of ions that occurs when a solid ionic compound dissolves.
NH42SO4 i 3 Calculate the boiling point of a 45 m solution of Na2SO4 in water assume 100 dissociation Kb H2O 052 Cm. Correct answer to the question Assuming 100 dissociation which of the following compounds is listed incorrectly with its vant Hoff factor i. Assuming 100 dissociation calculate the freezing point and boiling point of 186 m eqSnCl_4aq eq.
Calculate the value of i and estimate the percent ionization of HF in this solution. 1218 Which of the following aqueous solutions has the highest boiling point assume 100 dissociation for all soluble ionic compounds. Simply undo the crisscross method that you learned when writing chemical formulas of ionic compounds.
MgNO32 i 2 NH4NO3. 19Assuming 100 dissociation which of the following compounds is listed correctlywith its vant Hoff factor i. Assuming 100 dissociation which of the following compounds has an incorrectly listed vant Hoff factor.
Assuming 100 dissociation 10 mol of NaBr dissolved in 250 g of water Select have the same colligative properties as 10 mol Tonic compounds with polyatomic ions Molecular covalent compounds Olonic compounds with no polyatomic ions Acid and bases Question 5 1 pts If a certain mass of an ionic solute is added to a solvent such as water. For the same solution determine the vant Hoff factor assuming 100 ionization. K2SO4 i 3 b.
A Na2SO4 i 3 B NH4NO3 i 2 C Sucrose i 1 D Al2SO43 i 4 I know the answer is D but I could really use. Select all that apply. Assuming 100 dissociation which of the following compounds is listed correctly with its vant Hoff factor i.
CaClO32 i 2 NH42SO4 i 3 NH43PO4 i 5 Urea i 2 Sc2SO43 i 6. I - 5 Na2SO4 1-3. Assuming 100 dissociation which of the following compounds is listed correctly with its vant Hoff factor i.
Hence option B is correct. When NaCl dissociates completely it gives two ions Na and Cl. 3 2 i 2.
Sucrose i 1 d. Assuming 100 dissociation which of the following compounds is listed incorrectly with its vant Hoff factor i. It is important to be able to write dissociation equations.
Assuming 100 dissociation which of the following compounds is listed incorrectly with its vant Hoff factor I. The solution was found to freeze at -00943 C. Colligative constants can be found in the.
Therefore Vant Hoff factor is 2. When NaCl dissociates completely it gives two ions Naand Cl. Assuming 100 dissociation which of the following compounds is listed incorrectly with its vant Hoff factor i.
In which of the following solvents would you expect KBr to be most soluble. A 010m AlNO 3 3 D 018m NaCl B 011m Na 2 SO 4 E 035m C 6 H 12 O 6 C 015m K 2 CO 3 Ans. This result implies that A.
I - 2 Al2SO43. Therefore Vant Hoff factor is 2. NH4NO3 i 2 c.
Means How many mEq each molecule forms a.

Writing Chemical Formulae Crossover Method Teaching Resources Teaching Chemistry Chemistry Classroom Chemistry Lessons

Assuming 100 Dissociation Calculate The Freezing Point And Boiling Point Of 2 13 M Na 2so 4 Aq Study Com

Assuming 100 Dissociation Calculate The Freezing Point And Boiling Point Of 2 13 M Na 2so 4 Aq Study Com
Comments
Post a Comment